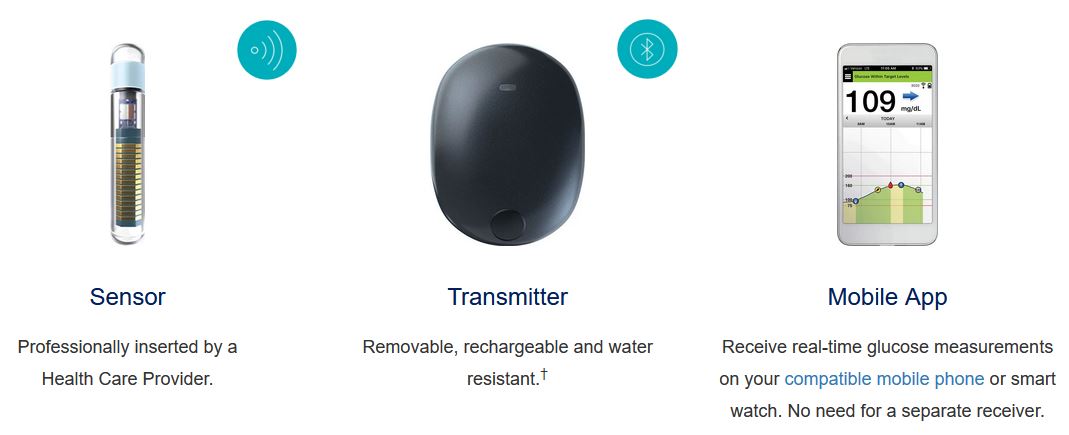
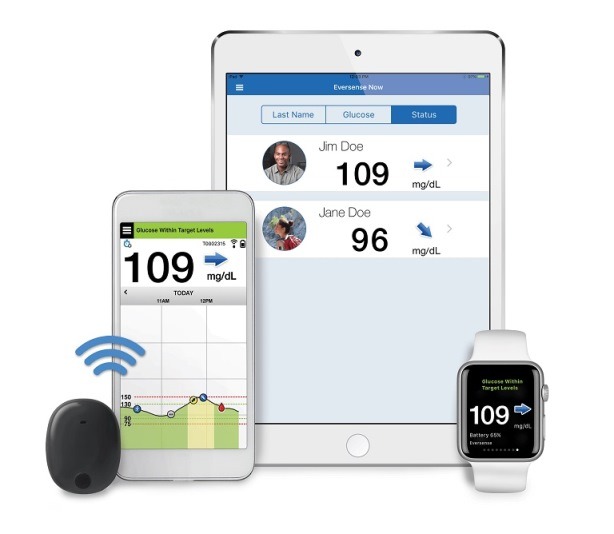
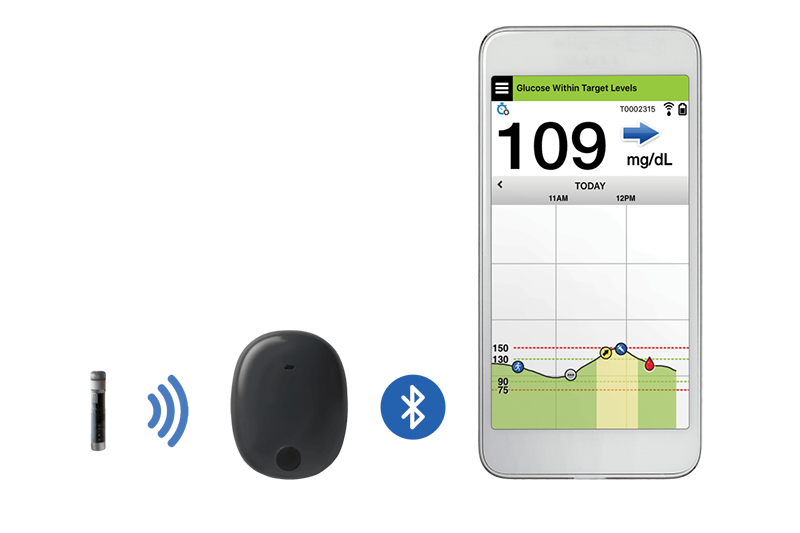
Eversense Implantable Glucometer Gets FDA Approval as Alternative to Finger Tests - 20451 Seneca Meadows Pkwy, Germantown, MD 20876, USA

Senseonics Receives FDA Approval To Expand Eversense CGM Certification To Nurse Practitioners, PAs | Medical Product Outsourcing

ASCENSIA DIABETES CARE ANNOUNCES FDA APPROVAL OF THE EVERSENSE E3 CONTINUOUS GLUCOSE MONITORING SYSTEM FOR USE FOR UP TO 6 MONTHS